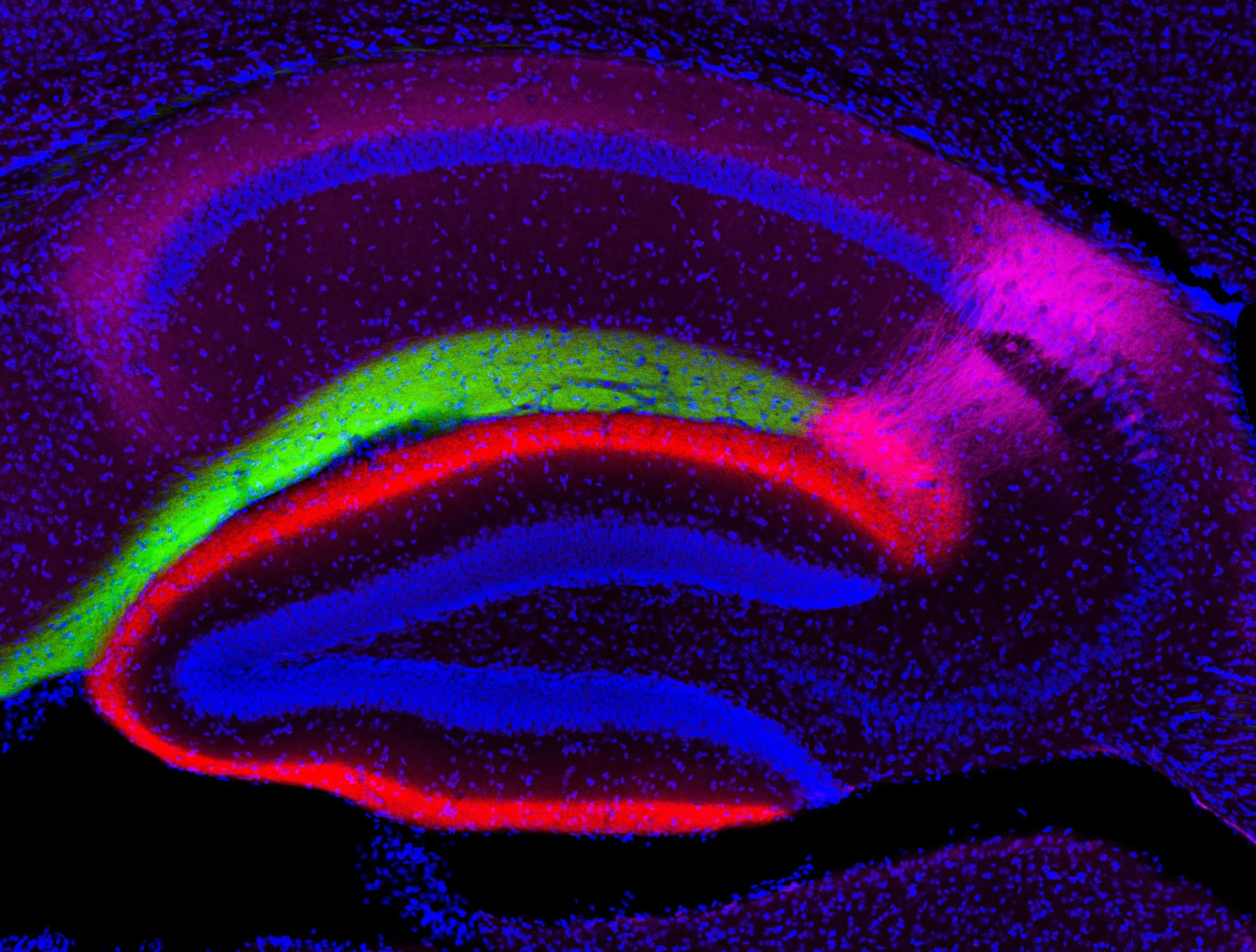
Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits.
Nature Neuroscience
Kohara K (1), Pignatelli M (1), Rivest AJ (1), Jung HY, Kitamura T, Suh J, Frank D, Kajikawa K, Mise N, Obata Y, Wickersham IR, Tonegawa S.
1 - These authors contributed jointly to this work
The formation and recall of episodic memory requires precise information processing by the entorhinal-hippocampal network. For several decades, the trisynaptic circuit entorhinal cortex layer II (ECII)-->dentate gyrus-->CA3-->CA1 and the monosynaptic circuit ECIII-->CA1 have been considered the primary substrates of the network responsible for learning and memory. Circuits linked to another hippocampal region, CA2, have only recently come to light. Using highly cell type–specific transgenic mouse lines, optogenetics and patch-clamp recordings, we found that dentate gyrus cells, long believed to not project to CA2, send functional monosynaptic inputs to CA2 pyramidal cells through abundant longitudinal projections. CA2 innervated CA1 to complete an alternate trisynaptic circuit, but, unlike CA3, projected preferentially to the deep, rather than to the superficial, sublayer of CA1. Furthermore, contrary to existing knowledge, ECIII did not project to CA2. Our results allow a deeper understanding of the biology of learning and memory.
Entorhinal Cortex Layer III input to the hippocampus is crucial for temporal association Memory.
Science
Science. 2011 Dec 9; 334(6061): 1415-20 Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S.
Abstract:
Associating temporally discontinuous elements is crucial for the formation of episodic and working memories that depend on the hippocampal-entorhinal network. However, the neural circuits subserving these associations have remained unknown. The layer III inputs of the entorhinal cortex to the hippocampus may contribute to this process. To test this hypothesis, we generated a transgenic mouse in which these inputs are specifically inhibited. The mutant mice displayed significant impairments in spatial working-memory tasks and in the encoding phase of trace fear-conditioning. These results indicate a critical role of the entorhinal cortex layer III inputs to the hippocampus in temporal association memory.
Characterization of Beta Amyloid Assemblies in Drusen: The Deposits Associated with Aging and Age-Related Macular Degeneration.
Exp Eye Res. 2004 Feb;78(2): 243-56
Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV.
Purpose: Recent studies strongly suggest that drusen, the extracellular deposits associated with age-related macular degeneration (AMD), are a manifestation of local inflammatory events. New evidence indicates that substructural elements within drusen contain activated complement components as well as amyloid beta (Abeta), a major pro-inflammatory components of Alzheimer's disease plaques. We characterized the ultrastructural organization and histochemical staining properties of these Abeta-containing elements in order to further assess their significance in drusen formation and AMD pathogenesis.
The Alzheimer's A Beta-Peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration.
Proc Natl Acad Sci USA. 2002 Sep 3;99(18): 11830-5
Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH.
Abstract:
Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss in older individuals worldwide. The disease is characterized by abnormal extracellular deposits, known as drusen, that accumulate along the basal surface of the retinal pigmented epithelium. Although drusen deposition is common in older individuals, large numbers of drusen and/or extensive areas of confluent drusen represent a significant risk factor for AMD. Widespread drusen deposition is associated with retinal pigmented epithelial cell dysfunction and degeneration of the photoreceptors cells of the neural retina. Recent studies have shown that drusen contain a variety of immunomodulatory molecules, suggesting that the process of drusen formation involves local inflammatory events, including activation of the complement cascade. Similar observations in Alzheimer's disease (AD) have lead to the hypothesis that chronic localized inflammation is an important element of AD pathogenesis, with significant neurodegenerative consequences. Accordingly, the amyloid beta (A beta) peptide, a major constituent of the neuritic plaques in AD, has been implicated as a primary activator of the complement in AD. Here we show that A beta is associated with a substructural vesicular component within drusen. A beta co-localizes with activated complement components in these "amyloid vesicles," thereby identifying them as potential primary sites of complement activation. Thus, A beta deposition could be an important component of the local inflammatory events that contribute to atrophy of the retinal pigmented epithelium, drusen biogenesis, and the pathogenesis of AMD.
Abstract
Declarative memory requires the integration and association of multiple input streams within the medial temporal lobe. Understanding the role each neuronal circuit and projection plays in learning and memory is essential to understanding how declarative and episodic-like memories are formed. This work here addresses the role of the medial entorhinal cortex layer III (MEC-III) to CA1 projections in episodic-like memory formation and recall. This circuit is addressed with a triple transgenic mouse which allows for the expression of tetanus toxin, an enzyme that disrupts synaptic vesicle fusion, specifically in MEC-III neurons. Utilizing this triple transgenic mouse model, which allows for the specific and reversible ablation of synaptic transmission only in medial entorhinal cortex layer III excitatory neurons, the function of this pathway in various learning and memory tasks is tested. Synaptic output from the medial entorhinal cortex layer III neurons is necessary for acquisition, but not recall of tone and contextual fear memories in trace fear conditioning, and not in delay conditioning. This is the first demonstration that acquisition and recall of the same memory engram do not require the exact same anatomy. Additionally, this pathway is necessary for performance in a delayed nonmatch-to-place working memory task, in which the animal must utilize memory from the previous trial to successfully complete the following trial. Both the trace and working memory paradigm require the integration of information across a delay, which we propose is supported by known persistent activity in entorhinal neurons. CAl receives input from both entorhinal layer III and CA3. We show that synaptic transmission from CA3 is not required for tone fear memory in the trace paradigm and not required for working memory in the same delayed nonmatch-to-place paradigm, further isolating the necessity for MEC-III inputs in both of these behaviors. Functional MEC-III synaptic transmission is also necessary for pattern-completion contextual recall in the pre-exposure contextual fear conditioning paradigm. Contrary to previous literature, the MEC-II to CAl pathway is not necessary for consolidation of spatial memories and anatomical tracings using this mouse line demonstrate that the MEC-III projects to CA1 and not CA3. The MEC-II pathway however, does project via two pathways to the same target in CA1, the perforant and alvear pathways. The alvear pathway has not been reported before in mice. Recent advances in mouse genetic tools have allowed for circuit studies of the medial temporal lobe. We have used these tools and elucidated some of the specific circuits involved with temporal and working memory.